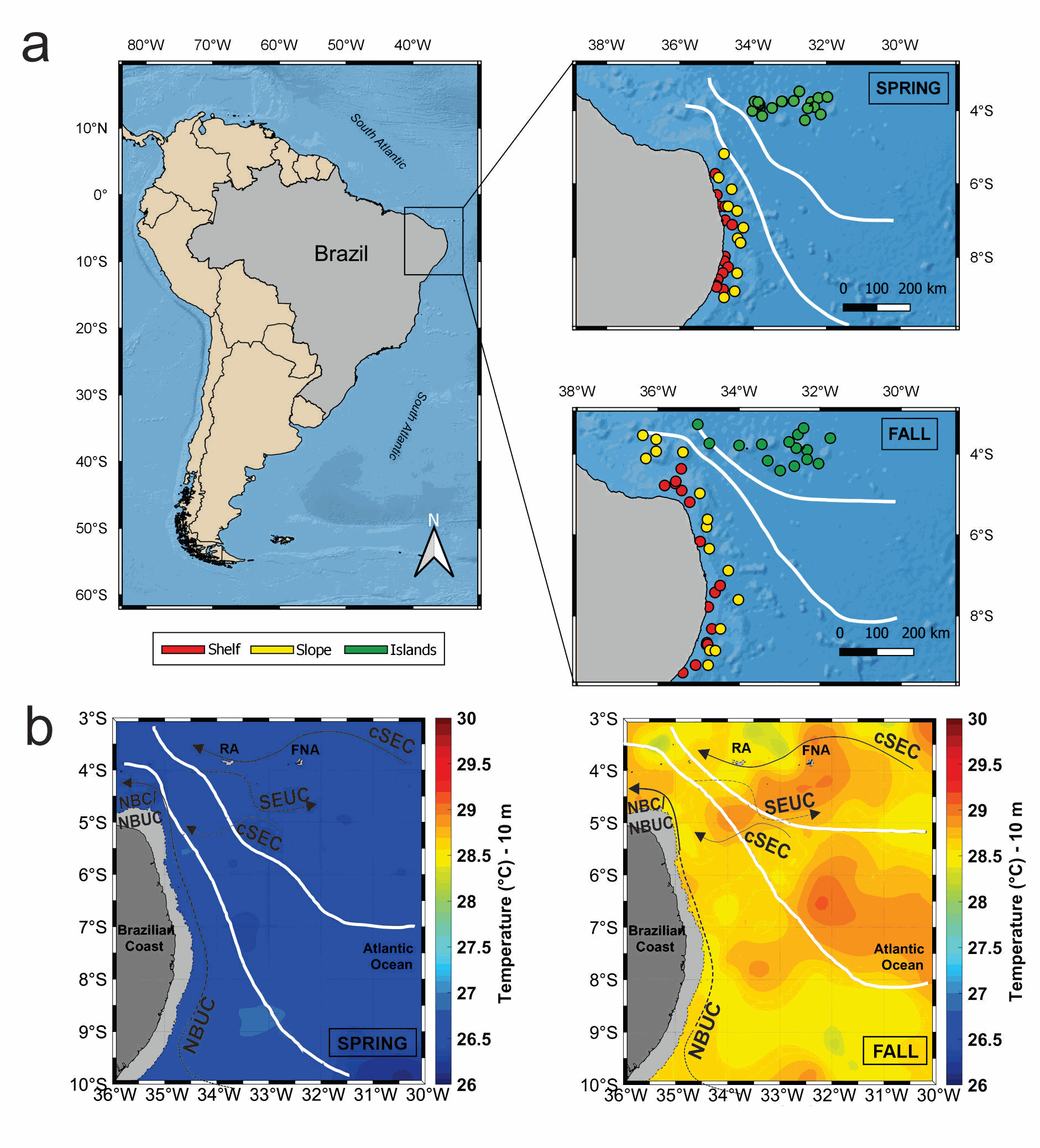
Date: 2015 Temporal extent: 2015-09 - 2017-05 Authors: Farias Gabriel Bittencourt, Ternon Jean-François, Hillion Sandrine , Melo Pedro Augusto Mendes de Castro, Carré Claire, Molinero Juan-Carlos, Baurand François, Grelet Jacques, Chaigneau Alexis, Costa da Silva Alex, Devasa Jeremy , Roubaud Fabrice, Bertrand Arnaud DOI: 10.17882/95171 Description: Samples were collected during the “Acoustics along the BRAzilian COaSt (ABRACOS)” oceanographic campaigns, carried out in Austral spring (30 August - 20 September of 2015 - ABRAÇOS1; Bertrand, 2015) and fall (9 April - 9 May of 2017 - ABRAÇOS 2; Bertrand, 2017) on board the French R/V ANTEA. Spring 2015 and fall 2017 are representative of canonical spring and fall conditions in terms of thermohaline structure and currents dynamics (Assunção et al., 2020; Dossa et al., 2021). Water sampling for pigments was carried out using a rosette at four depths defined by CTD profiles: Surface, Mixed Layer, Deep Chlorophyll Maximum (DCM) (which showed a mean depth of 100 m in spring and 80 m in fall) and at 200 m. In shelf shallow stations (< 50 m depth), where no peak of fluorescence was observed, the DCM and 200 m samplings were replaced by a sampling depth at ~10 m above the bottom. For each station and sampled depth, 500 mL of water were filtered in Whatman GF/F glass fiber for the estimation of pigment concentrations of the total phytoplankton community. Size fractionation of water samples was done using 20 μm filter meshes to distinguish the pico- and nanophytoplankton from total phytoplankton pigments. Microphytoplankton fraction (> 20 μm), was estimated by the difference between total and < 20 μm fractions. Filters were stored at 80 ◦C for subsequent HPLC pigment analysis. Chemotaxonomic analysis was carried out on an Agilent Technology 1200 series HPLC following the LOV Method described in Hooker et al. (2000), to assess phytoplankton biomass and diversity. Pigments were extracted in 100% methanol in the dark for 5 min at 4◦C. Samples were then sonicated and filtered on cellulose acetate filters to remove cell debris. A 600 μl aliquot was diluted with 150 μl Milli-Q water. For the analysis, 125 μl of this solution was taken and diluted in an injection loop with 125 μl of a 28 mM solution of Tetrabutyl ammonium acetate. The pigments were then separated on a ZORBAXEclipse XDB-C8 column from Agilent Technology, with 3 mm in diameter,150 mm in length and 3.5 μm in porosity. The column temperature was maintained at 60 ◦C and the flow rate at 0.55 ml min 1. The separation was based on a linear gradient between a solution of methanol/Tetrabutyl ammonium acetate 28 mM, 70:30 (v/v), and a 100% solution of methanol. Chlorophyll-a + Divinyl Chlorophyll-a were used to determine total phytoplankton biomass (TChl-a), while all other pigment markers allowed identifying major algal groups (Table 1). The HPLC system was calibrated with external standards (DHI Water and Environment, Horsholm, Denmark).